Please note what I mentioned before, frugality and over saturation do not combine. By frugality, I mean ridiculously frugal, these ingredients are not expensive, Epsom salt, sugar? Live a little, please!
What is done is done though, for now, one day in the future we will try and succeed in these experiments. Like Strawberry Shortcake says, "You can't not try." Which I roughly translate to you have to try and try and try and try and then try some more. Then give up for a couple days and go back and try again. My blind confidence that things will work has always lead me to interesting results.
Growing Rock Crystals!
Rock crystals, if you haven't heard of them, are sugar crystals, they are a great example of over saturation of sugar in water. I was a little hesitant about having a bunch of super sugary water hanging around the house. Not because the cat, dog, husband or Aimee might drink it, but because a long time ago I heard it attracted cockroaches....I just googled this theory, I couldn't find anything. It says they are attracted to sugar and drink water, so I am sure they wouldn't mind a time saving combination of the two. WE did not have any problems or attract any kinds of bugs with this experiment.
I grew rock crystals once when I was a kid and I have had them multiple times before. I do remember the growing being somewhat successful and eating them being somewhat delicious. The are supposed to end up looking something like this;
Layers upon layers built up on each other. Beautiful. Delicious. Crystallized sugar on a stick, what more could you want? Since I hadn't made these in so long I looked around the Internet for some directions/instructions. I ended up using the about.com version.
First you start with; sugar, water, two glasses or cups, string (cut long enough so it is not touching the sides or the bottom) food coloring and pencils. Mistake! I wasn't really thinking about it and used glitter pencils. That is fine, however most glitter contains a teeny, tiny, almost, but really not enough to worry about, amount of lead in it. After I realized this I knew we would not be eating these crystals. SO please use a regular old fashioned #2. Or a Popsicle stick. Or anything that will hold the string.
We used the sugar and cream brand of string. It is a cotton string with a nice ply. We had some laying around from a project I am making Aimee. I thought the name fitting.
Dissolve the sugar in the water, stirring it constantly and bring it just to a boil. Don't over cook it or else it will get too hard. We added the food coloring after it was done cooking, but before we cooled it. You have to cool it for a long time. I took pictures, but then decided it would be best not to post pictures of the inside of my refrigerator.
After the sugar/water solution is cool you need to dip your string in, to promote a starter crystal to form on your string.
The liquid is pretty thick. So we found it a little hard to keep the string straight. About.com suggested using something to weight your string, but of course something non-toxic like a life-saver candy. We didn't have any. I am not sure if I count this as a mistake. We separated the solution, mainly because all of it wouldn't fit into one cup and coloring one pink and one yellow.
Cover with a coffee filter or something similar and wait. And wait. And wait. Usually it is only supposed to take a couple of days.
water solution. You can go ahead and take these out with a spoon. This happened after a few days with both of our glasses.
After a couple more days we noticed that crystals had started forming on the sides and bottom of our container. The instructions noted to transfer to a new clean cup if this started to happen. The good news is we started to see lots of activity on our strings. We definitely got a starter crystal going. The bad news, our cups had crystallized in the worst way. I wish I could tell you what mistake I made, so you would be so fortunate not to make the same one, but I have no idea what I did wrong. I figured it was just the way it was and we made a new batch of sugar water, since we didn't have enough after the crystallizing.

 That is a hot mess of crystals. The even worse news, we didn't have a dishwasher, so these babies had to soak and soak and soak. That should be a requirement for this experiment. String, glasses, dishwasher, sugar, etc.
That is a hot mess of crystals. The even worse news, we didn't have a dishwasher, so these babies had to soak and soak and soak. That should be a requirement for this experiment. String, glasses, dishwasher, sugar, etc. Here is our second batch of mix. We tried to even things out this time. We put the mix and strings with starter crystals on them in clean cups and waited again.
Even though the crystals did get a lot bigger, they were not forming as well as I thought they should. There wasn't any of the BIG rock type formation I was expecting. It was very grainy and very small. This time the crystallization in the cups worsened. The entire liquid, except for the small portion on the right was all crystallized and stuck to the glass worse then the first time. It happened with the yellow also.
After this, I was unsure of what to do. Not willing to quite give up, but definitely not willing to make another batch of the sugar water mixture (we had already used six cups, almost a whole bag) we put the strings in the remaining clear mixture and waited a little longer.
Amazingly enough, after about the same amount of time as the previous cups, there wasn't as much crystallization in the cups. A little on the bottom and a little crust on the top. However the crystals on the top part of the string did start to degrade and come apart. Sigh.
Not too bad. I guess. After this though, we called it, well not quits, but we left the experiment open. Hopefully to come back to it at a later time when we are a little older and wiser. Then we will make awesome huge rock crystals. Instead of these.
All things considered, it was pretty cool that something did form on the strings. Looking at the pictures, they do look pretty cool. I think when I wasn't looking Aimee might have snuck in a lick, but otherwise, we mainly just looked at them for a couple of days. This experiment took a while and I wasn't sure how I felt about her eating them after how long they had been sitting out.
It is definitely an experiment worth trying again and again and again.
Stalactites and Stalagmites
Our next experiment came from one of our books. It looked pretty easy and pretty cool so I thought we could give it a try.
Here are the ingredients.
2 jars, water, epsom salt 16 oz., a spoon, scissors, 2 nails, a pan or sturdy paper plates, yarn.
Start by filling the jars with water and then adding the epsom salt a spoonful at a time. Until you literally can not add any more, it just will not dissolve. PLEASE ADD MORE THEN YOU THINK IS, WAY MORE THEN ENOUGH. Mistake! I stopped once I got through a 1/4 pound of salt for each glass. In other words, I only used half of the box for both jars. I probably could have and should have doubled that amount.
Next tie one nail to each end of the yarn/string. You want it to be long enough to drape over close (about a couple of inches above), but not touch the plate. Put the one nail with the string in each jar and adjust the distances. Now wait.
Mistake! I used a thick yarn that I had laying around the house, I think this yarn would have been okay, had it not been so wavy. I believe the whole string is supposed to get wet, though ours never did. If you look at the picture in the book, the string they use is pretty thick. Also the length/distance of the string and that above the plate is different then the directions specified.
We waited for a 3 or 4 days and almost nothing happened. Our plate did get wet, the bottom of the string was wet, but the whole string wasn't. Dried epsom salt showed up on the edges of the jars and the plate, but that was about it. According to the book a lot was supposed to be forming at this point. A little beside myself with frustration, I blamed the string and we tried again.
Our second attempt yielded even less of a result. We kept it up for a little longer and still nothing occurred. This is when I decided the problem was mainly the lack of epsom salt. We will definitely do it again and use a lot more epsom salt, I mean honestly, it is like .89 cents for a pound. For pete's sake.
I think it is evident how I felt about crystal week, but what about Aimee? She had fun for the most part, she loved the Dora experiments the most. Everything else was a little or a lot above her. She did a great job paying attention and tried really hard, but the lack of results combined with the over complecity of the whole thing was a little much. She hasn't talked about it as much as she has the other weeks that we have done.
Happy themeing!
More weeks of fun to come soon!























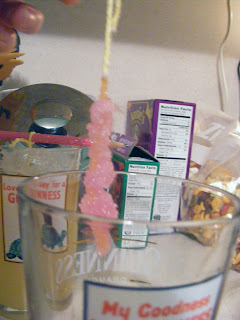

















No comments:
Post a Comment